Gain a comprehensive understanding of cell therapy advancements with a detailed analysis of these abstracts. Furthermore, we invite you to download the list of abstracts by clicking on Download Abstracts Summaries.
Please note that some information has been redacted from the downloadable content. If you would like to receive the complete version and speak with one of our cell therapy specialists, please do not hesitate to contact beacon@hansonwade.com to discuss your drug development data needs.
ASH 2023 Abstract Analysis
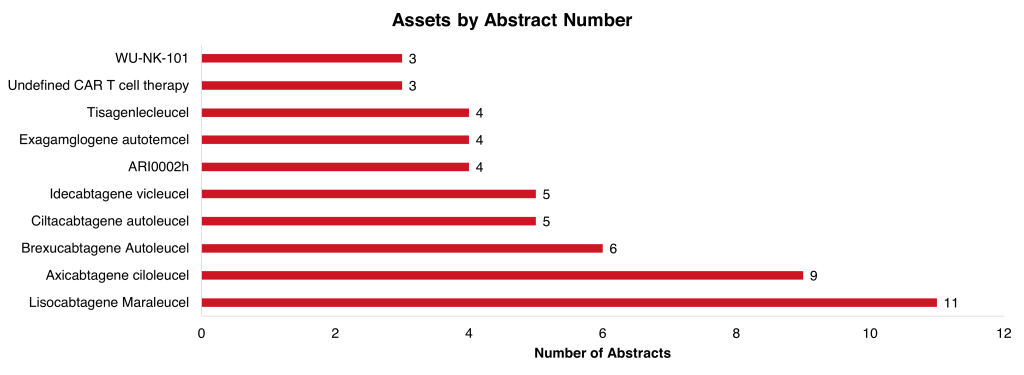
The most commonly researched drug was lisocabtagene maraleucel. It is a CAR T therapy manufactured by Bristol-Myers Squibb. This drug had 11 clinical abstracts, 9 trial results, and two pooled analyses, including one health care resource utilization analysis. The data was collected from the TRANSFORM (NCT03575351) and PILOT (NCT03483103) studies. Additionally, there were three abstracts for WU-NK-101, which included two preclinical results and one trial outline. However, the phase 1/2 study for WU-NK-101 was terminated in February 2023 due to insufficient funding/staff. The trial outline did provide information about the patient lymphodepletion and dosing regimens.
Therapeutic Class Breakdown
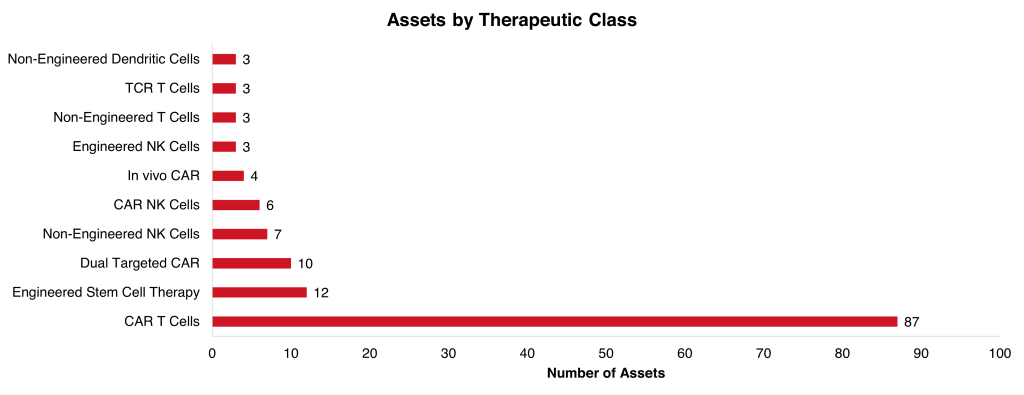
Removing assets mentioned in multiple abstracts and counting them only once leaves 134 assets for analysis. The most common therapeutic class was CAR T cells comprising 65% of the total. The second most common therapeutic class was engineered stem cell therapies, including Cimeio Therapeutics CD33 HSPCs that were identified from ASH, and RM-001 that had pooled analysis presented. There were also 4 in vivo CAR assets that had results presented: three from Umoja Biopharma (UB-VV100, UB-VV111, and UB-VV200) and one from Sana Biotechnology (SG299).
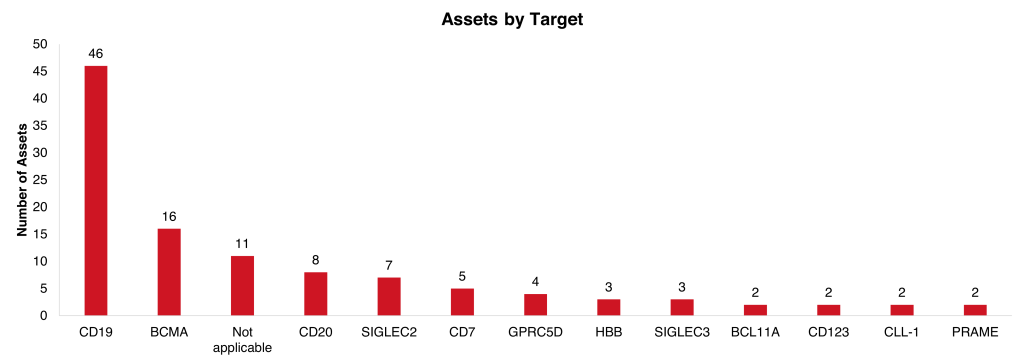
The most common targets are CD19 and BCMA, the most well-established and validated targets which reflects the overall cell therapy space with CD19 being targeted by 614 assets, and BCMA targeted by 201 assets. Other targets only mentioned by one asset include MSLN from OBP-101, NKG2D from NKX101 and BAFF-R from PMB-101. OPB-101 is the lead asset from Outpace Bio and expects an IND by 2H 2024.
Trial Phase Distribution
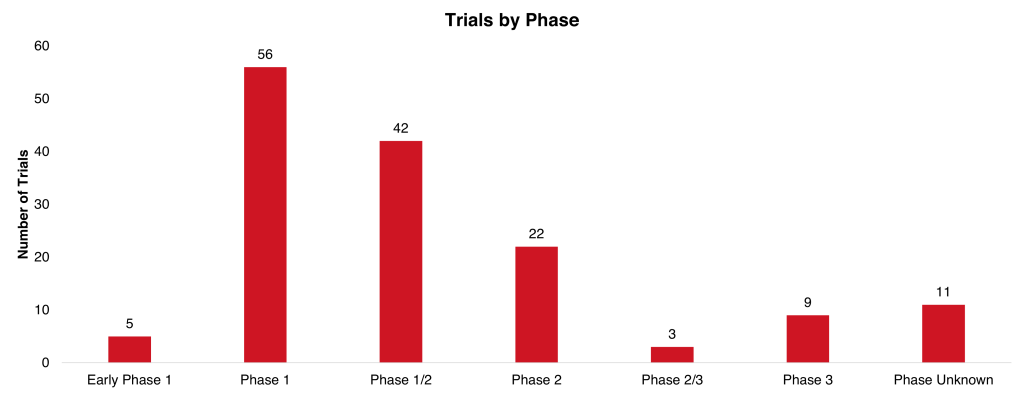
Trials mentioned in multiple abstracts have been counted once, with a total of 148 trials mentioned in the 155 clinical abstracts. The most common trial phase is phase 1 which is 36% of total trials. Results were presented for actalycabtagene autoleucel, which was approved by the CDSCO in October 2023 as India’s first CAR T cell therapy.
The 2023 ASH Annual Meeting provided a comprehensive snapshot of the evolving cell therapy landscape. We provide you with a downloadable abstract list that serves as a valuable resource for further exploration and research. You can download the comprehensive list by clicking Download Abstracts Summaries for a detailed exploration of the abstracts discussed.
Please note that some information has been redacted from the downloadable content. If you would like to receive the complete version and speak with one of our cell therapy specialists, please do not hesitate to contact beacon@hansonwade.com to discuss your drug development data needs.
Beacon Cell Therapy
How the most complete cell therapy database can help you
What we cover
Beacon Cell Therapy sector-specific curated database includes trial and drug records for preclinical, active, approved, and discontinued:
- Receptor-engineered cell therapies such as CAR (chimeric antigen receptor) and TCR (T-cell receptor) therapies
- Other engineered or non-engineered immune cell therapies such as natural killer cells and regulatory T cells (Tregs)
- Stem cell-derived and iPSC-derived cell therapies such as immune cells, somatic cells (e.g. cardiomyocytes and retinal pigment cells) and precursor cells
How Beacon Cell Therapy works
Search the clinical trial and drug landscape with over 15 filters, including cell type, cell source, and starting material, enabling you to extract the data you need to conduct complex analyses efficiently.
Our unique Milestones filter and visualization highlights past, present, and future drug development milestones, including drug and trial readouts, asset history, and regulatory announcements. This enhancement provides an accurate, timely and exhaustive single-drug timeline allowing you to benchmark progress in the cell therapy space.
Find out more
Beacon is the essential decision-support tool for developers of complex therapeutics. Our market-defining, proprietary ontologies, combined with the most accurate and comprehensive life sciences data, provide our customers with unparalleled visibility of the drug and trial landscape.
With Beacon, you can make drug development decisions with confidence. This is why 23 of the world’s top 25 drug developers trust us.
At Beacon, we are committed to empowering our pharmaceutical and biotech industry partners with accurate, comprehensive data. We are not just a tool; we see ourselves as a partner in the drug development journey. Contact us to learn how we help you.


